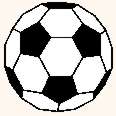
 C60 is a molecule that consists of 60 carbon atoms,
arranged as 12 pentagons and 20 hexagons. The shape
is the same as that of a soccer ball: The black pieces of leather are the
pentagons, the hexagons are white. There are 60 different points where three
of the leather patches meet. Imagine a carbon atom sitting at each of these
points, and you have a model of the C60 molecule. That
model, however, is vastly out of scale: If the
C60 molecule were the size of a soccer ball, then the
soccer ball in turn would be roughly the size of the earth.
C60 is a molecule that consists of 60 carbon atoms,
arranged as 12 pentagons and 20 hexagons. The shape
is the same as that of a soccer ball: The black pieces of leather are the
pentagons, the hexagons are white. There are 60 different points where three
of the leather patches meet. Imagine a carbon atom sitting at each of these
points, and you have a model of the C60 molecule. That
model, however, is vastly out of scale: If the
C60 molecule were the size of a soccer ball, then the
soccer ball in turn would be roughly the size of the earth.
The most striking property of the C60 molecule
is its high symmetry. There are 120 symmetry
operations, like rotations around an axis or reflections in a plane, which
map the molecule onto itself. This makes C60 the molecule
with the largest number of symmetry operations, the most symmetric
molecule.
Based on a
theorem of the mathematician
Leonhard Euler, one can show that a spherical
surface entirely built up from pentagons and hexagons must have exactly
12 pentagons.
Depending on the number of hexagons, molecules of different sizes are obtained.
 They are called Fullerenes, after the American architect
Richard Buckminster Fuller.
Fuller, who is shown here on the cover of Time Magazine of January 10, 1964,
was renowned for his geodesic domes, that are based on hexagons and pentagons.
An even earlier example of such a construction was the dome of the first
planetarium, built by Zeiss
in 1922.
They are called Fullerenes, after the American architect
Richard Buckminster Fuller.
Fuller, who is shown here on the cover of Time Magazine of January 10, 1964,
was renowned for his geodesic domes, that are based on hexagons and pentagons.
An even earlier example of such a construction was the dome of the first
planetarium, built by Zeiss
in 1922.
It should come as no surprise that a shape as symmetric and beautiful as
that of the C60 molecule, has occupied many artists
and mathematicians over the centuries. Probably it was already known to
Archimedes (after all, it's one of the
Archimedean solids!), although no drawings seem to have survived.
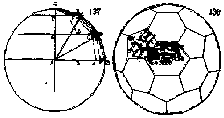 The oldest known picture of the soccer-ball-shape seems to be a drawing
found in the
Vatican library.
It is from a book of the painter and mathematician
Piero della Francesca and dates from the
1480s.
The oldest known picture of the soccer-ball-shape seems to be a drawing
found in the
Vatican library.
It is from a book of the painter and mathematician
Piero della Francesca and dates from the
1480s.
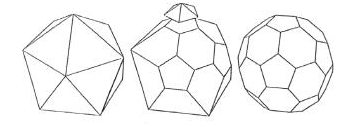
 Johannes Kepler coined the name truncated
icosahedron for this shape. An example of the appearance of the truncated
icosahedron in art is shown in this picture from an Italian cathedral. At the
top we see an icosahedron. It is bounded by twenty equilateral triangles. At
each of the 12 vertices of the icosahedron, five of the triangles meet. Cutting
off ('truncating') these vertices thus replaces each of them by a pentagonal
face; it also converts each of the twenty former triangular faces into a
hexagon. We can see the resulting truncated icosahedron at the bottom of the
picture. This is the shape of the C60 molecule.
Johannes Kepler coined the name truncated
icosahedron for this shape. An example of the appearance of the truncated
icosahedron in art is shown in this picture from an Italian cathedral. At the
top we see an icosahedron. It is bounded by twenty equilateral triangles. At
each of the 12 vertices of the icosahedron, five of the triangles meet. Cutting
off ('truncating') these vertices thus replaces each of them by a pentagonal
face; it also converts each of the twenty former triangular faces into a
hexagon. We can see the resulting truncated icosahedron at the bottom of the
picture. This is the shape of the C60 molecule.
truncating an icosahedron:
The C60 molecule was discovered by Harold Kroto,
James Heath, Sean O'Brien, Robert Curl, and Richard Smalley in 1985
(Nature 318, 162).
The group actually tried to understand the absorption spectra of interstellar
dust, which they suspected to be related to some kind of long-chained carbon
molecules. Unfortunately they could not solve that problem. But their work was
not completely unsuccessful, since in the course of their experiments they
discovered the Buckyball which generated so much excitement among scientists
and won Curl, Kroto, and Smalley
the 1996
Nobel prize in chemistry.
Initially, C60 could only be produced in tiny
amounts. So there were only a few kinds of experiments that could be
performed on the material. Things changed dramatically in 1990, when Wolfgang
Krätschmer, Lowell Lamb, Konstantinos Fostiropoulos, and Donald
Huffman discovered how to produce pure C60 in
much larger quantities (Nature 347, 354). This opened up completely
new possibilities for experimental investigations and started a period of
very intensive research. Nowadays it is relatively straightforward to
mass-produce C60. For instructions check the
Workshop on how to
produce bulk quantities of pure C60
or have a look at the
research
page of two undergraduates who produced C60.
If you don't want to do the lab work yourself, you can also
buy
C60
(prices).
The discovery of C60 has stimulated a large activity
in chemistry. It opened up the new branch of
Fullerene-Chemistry
which studies the new families of molecules that are based on Fullerenes.
By 1997 about 9000
Fullerene
compounds were known.
C60 molecules condense to form a solid of weakly
bound molecules. This crystalline state is a new form of solid carbon,
besides the long known diamond and graphite. It is called Fullerite.
Much of the work in physics is centered around the solid phases of
C60. If certain alkali atoms (A) are added to solid
C60, new compounds like
A3 C60 can be formed, the
alkali-doped Fullerides. If A is
potassium (K) or
rubidium (Rb)
the compounds are superconductors. That means that below a certain temperature
Tc they conduct electric currents without any
resistance. For the alkali-doped Fullerides Tc is
quite large (20-40 K) compared to "conventional" superconductors.
At a very early stage the British House of Lords discussed the Fullerenes.
The final part of the transcript of the hearing is quite entertaining!
If you are more interested in
applications
of C60, have a glimpse at the
Fullerene
Patent Database.
You can find more information on the Fullerenes in:
Back to main page.
|
Andersen Group
|
Max-Planck-Institut für Festkörperforschung
Heisenbergstraße 1 D-70569 Stuttgart
|
 C60 is a molecule that consists of 60 carbon atoms,
arranged as 12 pentagons and 20 hexagons. The shape
is the same as that of a soccer ball: The black pieces of leather are the
pentagons, the hexagons are white. There are 60 different points where three
of the leather patches meet. Imagine a carbon atom sitting at each of these
points, and you have a model of the C60 molecule. That
model, however, is vastly out of scale: If the
C60 molecule were the size of a soccer ball, then the
soccer ball in turn would be roughly the size of the earth.
C60 is a molecule that consists of 60 carbon atoms,
arranged as 12 pentagons and 20 hexagons. The shape
is the same as that of a soccer ball: The black pieces of leather are the
pentagons, the hexagons are white. There are 60 different points where three
of the leather patches meet. Imagine a carbon atom sitting at each of these
points, and you have a model of the C60 molecule. That
model, however, is vastly out of scale: If the
C60 molecule were the size of a soccer ball, then the
soccer ball in turn would be roughly the size of the earth.